basic MCQ on the basis of ray optics:-
Latest Posts
Identification Of Enantiomers and Diastereomers ......
Identify Enantiomers and Diastereomers and Identical Isomers is Very Confusing to Identify From Fischer projection .....
Today I Give You a super Trick any question You Solved in 30sec...
super Trick video is also given in below, You can watch these also. You never forget or confuse in your Whole life to identify Enantiomers and Diastereomers....
By Bookage Definition Of Enantiomers and Diastereomers is
Enantiomers:- Stereoisomers having Mirror image relationships, and not super impossible to each other, is called Enantiomer.
But when This type of Structure is Given Then You Confuse How to Find out Mirror Image Relationship..
Diastereomers:- Stereoisomers having no mirror image relationship and superimpossible to each other, is called Diastereomers.
confusing structure Just like this.
#Super_Trick Read carefully...
Note:- Before Staring Trick You keep in Mind Two-term
1) Same Configuration:- means Both compound Chiral Center is Either R-configuratio or S-configuration.
2) Different Configuration:- One Compound chiral is R-configuration then another compound configuration is S. both are diffrent.
1 Chiral Center Trick:-
- If Configuration is Same, then it's a---- Identical Isomers
- If Configuration is Different, then it's a---- Enantiomers
Note:- In one Chiral Center Diastereomers Not possible....
2 Chiral Center Trick:-
- If Configuration is Same, then it's a---- Identical Isomers
- If Configuration is Different, then it's a---- Enantiomers
- If One Chiral center configuration is same another is different---> Diastereomers
This practice Set for Physics Contain 10 Questions..
Each question has four options. Only one of these options is the correct answer.
1) A parallel plate capacitor has a capacitance of 50µF in air and 110µF When immersed in oil, the dielectric constant of oil is --
(a) 0.49 (b) 0.55
(c) 1.10 (d) 2.20
2) Two spheres of radii R1 and R2 joined by a fine wire are raised to a potential V. Let the surface charge densities at these two spheres be respectively σ1 and σ2. Then--
(a) 200/3V (b) 100/3V
(c) 400/3V (d) 80/3V
4) Find the Charge stored in the capacitor?
(a) 10 µC (b) 20 µC
(c) 30 µC (d) 40 µC
5) A 1 µF capacitor and 2 µF capacitors are connected in series across a 1200V supply line. The charge and Voltage across 1µF capacitor.
(a) 800 µC and 400V
(b) 800 µC and 800V
(c) 400 µC and 400V
(d) 400 µC and 800V
6) Two capacitors each having capacitance C and break down voltage V are joined in series. The Capacitance and breakdown voltage of the combination will be?
(a) 2C and 2V
(b) 0.5C and 0.5V
(c) 2C and 0.5V
(d) 0.5 µC and 2V
7) Two metal spheres of radii a and b are connected by a thin wire. Their separation is very large compared to their dimensions. The capacitance of the system is
8) A charge Q is divided into two parts of q and Q-q. If the Coulomb repulsion between them when they are separated is to be maximum, the ratio of Q/q should be?
(a) 2 (b) 0.5
(c) 4 (d) 0.25
9) In the Circuit shown the current through 2ohm resistance is?
(a) 5A (b) 2A
(c) 0A (d) 4A
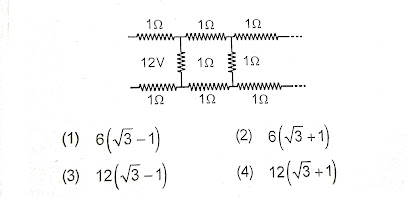
10) In the following fig. the current drawn by the battery of 12V supply (in amp) will be-
Facebook page question Solution..
Super Trick Find Bond Order in 5 sec.
Hey Everyone,
I make a Super Trick find Bond Order in just 5 sec.
Generally, all of You Find Bond Order by making energy diagrams, but this method is too time-consuming and in competitive Exams, so this reason I make this super trick video.
If you want a Video of This Trick Then You Click on BOND ORDER
Generally This Trick applicale on total electron number 8 To 20.
Super Trick
This trick is very easy you remember two thing
1) When the total electron number of an atom is 8 then its Bond Order is Minimum, which Means its Bond Order Valu is Zero.
2) When the total electron number of an atom is 14 then its Bond Order is Maximum, which Means its Bond Order Valu is 3.0
when the electron number of an is 8 then its Bond Order is Increase by 0.5 up to Electron number 14,
After Electron Number 14 Bond Order decrease by 0.5 and Electro number 20 Bond Order is zero, which means Minimum.
NOTE:- One Thing Remember 8 to 14 electron Number, electron go in Bonding Molecular Orbital and 14 to 20 electron Number, electron go in Anti Bonding Molecular Orbital.
Top practice question on Electrochemistry:-
1) The charge required for reduction of 1 mole of MnO4- ions into Mn2+ ions is-
2) The pH of 0.5L of 1.0 M NaCl after the electrolysis for 965 s using 5.0A current is-
E-Z NOMENCLATURE:-
E-Z Nomenclature:-
- In nomenclature Cis or Trans Term is not allowed.